Abstract
Chimeric antigen receptor (CAR) T-cell therapies have transformed the treatment landscape in relapsed or refractory B-cell malignancies and multiple myeloma. However, the risk of life-threatening complications such as cytokine release syndrome (CRS) and immune effector cell-associated neurotoxicity syndrome (ICANS) have led many centers to integrate CAR T-cell infusion as an inpatient procedure. Few reports document the implementation of an entirely outpatient CAR T-cell therapy program or the impact on patient outcomes. Here, we share our experience in the development and implementation of an outpatient model for CAR T-cell therapies. We describe a systematic approach for coordination of care, allocation of resources, education of staff, patients and caregivers, and toxicity management to make CAR T-cell therapies more accessible on an outpatient basis.
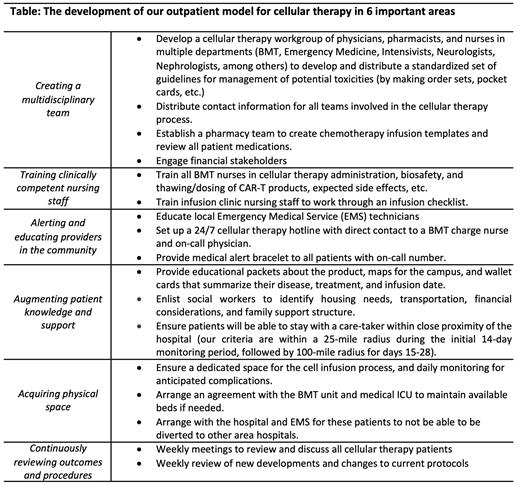
Development of our outpatient CAR T-cell therapy program began in 2017 with the first outpatient CAR T-cell infusion in September 2019. The Table summarizes key aspects of our outpatient program with a focus on 6 important areas - creating a multidisciplinary team, training clinically competent nursing staff, alerting and educating providers in the community, augmenting patient knowledge and support, acquiring physical space, and continuously reviewing outcomes and procedures. Outpatients were monitored after cell infusion with daily provider visits on days 1-14 then three times per week on days 15-28. Response was assessed using the Lugano response criteria for all lymphoma patients by Positron emission tomography (PET) scan at 1 and 6 months, and by bone marrow biopsy and minimal residual disease (MRD) testing for acute lymphoblastic leukemia (ALL) patients.
From September 2019 to May 2021, 20 patients (19 adults and 1 child) received commercial CAR T-cell products: 13 axicabtagene ciloleucel (axi-cel) for diffuse large B-cell lymphoma (DLBCL), 6 tisagenlecleucel (tisa-cel) (3 for ALL and 3 for DLBCL), 1 brexucabtagene autoleucel (brex-cel) for mantle cell lymphoma. Eighteen patients (90%) received outpatient infusions. Two patients receiving axi-cel were infused while inpatient based on physician consensus for high disease burden and co-morbidities. Of the 18 outpatients, 5 (28%) were admitted within 72 hours and 14 (78%) within 30 days after infusion. No patient required a visit to the emergency department. Median days to admission was 4 (range 1-28) and median days admitted was 9 (range 1-30). Indications for admission were fever in 12 cases (86%) and neurological symptoms in 2 cases (14%). Of 12 (67%) patients with CRS, 1 had grade 3. Of 6 (33%) patients with neurotoxicity, 1 had grade 3. Seven patients (39%) received an IL-6 inhibitor with a median of 1 dose (range 1-5) and 7 received dexamethasone for a median duration of 4 days (range 1-21). There were no treatment-related deaths among those infused as outpatients. At one month, the overall response rate (ORR) was 82% (9 of 11 patients) with axi-cel (6 complete responses (CR) and 3 partial responses (PR)), 100% with tisa-cel (5 CR and 1 PR; all 3 ALL patients were MRD negative) and a CR in 1 patient with brex-cel. At 6 months, 50% (4 of 8; 3 have not reached the 6-month time point yet), 75% (3 of 4) and 100% (1 of 1) of these patients continued to respond with axi-cel, tisa-cel, and brex-cel, respectively. Early outcomes are comparable to those seen in pivotal clinical trials (ZUMA-1/2, ELIANA, and JULIET). Outpatient administration and management of CAR T-cell therapy was safe and feasible with our approach. With the ongoing expansion of new indications for CAR T-cell products, strategies for safe outpatient administration are relevant to the practical clinical utility of these therapies.
Ibrahimi: Karyopharm Theraputics: Divested equity in a private or publicly-traded company in the past 24 months. Wieduwilt: Gilead: Membership on an entity's Board of Directors or advisory committees; Reata: Current holder of stock options in a privately-held company.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal